Graphene

If the 20th century was the age of plastics, the 21st century seems set to become the age of graphene—a recently discovered material made from honeycomb sheets of carbon just one atom thick. Science journals have been running out of superlatives for this wondrous stuff: it's just about the lightest, strongest, thinnest, best heat- and electricity- conducting material ever discovered. And if we're to believe the hype, it promises to revolutionize everything from computing to car tires and solar cells to smoke detectors. What is this strange and remarkable new stuff? Let's take a closer look!
Photo: A pencil like this is a wooden shaft filled with a stick of soft graphite, a type of carbon made from strongly bonded layers of atoms that are very weakly held together by Van der Waals forces. As you drag your pencil along the page, the thin layers of graphite shear off and stay behind, making the black line you can see. Now if you could shave off a super-thin layer of graphite, just one atom high, what you'd have would be graphene. There are tiny specks of graphene in any pencil mark like this, but since they're only one atom high, you'll be doing well to spot them!
What is graphene?
In school you probably learned that carbon comes in two basic but startlingly different forms (or allotropes), namely graphite (the soft, black stuff in pencil "leads") and diamond (the super-hard, sparkly crystals in jewelry). The amazing thing is that both these radically different materials are made of identical carbon atoms. So why is graphite different to diamond? The atoms inside the two materials are arranged in different ways, and this is what gives the two allotropes their completely different properties: graphite is black, dull, and relatively soft (soft and hard pencils mix graphite with other materials to make darker or fainter lines); diamond is transparent and the hardest natural material so far discovered.
If that's what you learned in school, you probably finished your studies quite a while ago, because in the last few years scientists have discovered various other carbon allotropes with even more interesting properties. There are fullerenes (discovered in 1985; hollow cages of carbon atoms, including the so-called Buckyball, Buckminsterfullerene, made from a kind of football-shaped cage of 60 carbon atoms), nanotubes (discovered in 1991; flat sheets of carbon atoms curled into amazingly thin, hollow tubes one nanometer in diameter)—and (drum roll) graphene (discovered in 2004).
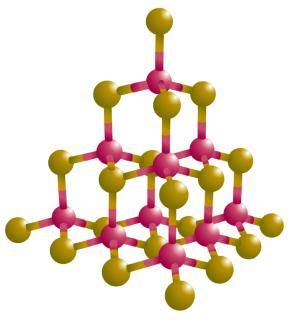
Artwork: Right: Diamond has a 3D (three-dimensional) crystal lattice that looks a bit like this (which is actually cadmium sulfide). The blobs are the atoms and the lines are the bonds that join them together. Bonds are invisible, but we draw them like this so we can visualize them more easily. Picture by courtesy ofNASA Marshall Space Flight Center (NASA-MSFC).
So what exactly is graphene? Peer inside lots of familiar solid materials (including most metals) and you'll find what's known as a crystal lattice (another name for a solid's internal, crystalline structure): lots of atoms arranged in a regular, endlessly repeating, three-dimensional structure a bit like an atomic climbing frame, only instead of bars there are invisible bonds between the atoms that hold them together. Diamond and graphite both have a three-dimensional structure, though it's completely different: in diamond, the atoms are tightly bonded in three-dimensional tetrahedrons, whereas in graphite, atoms are bonded tightly in two-dimensional layers, which are held to the layers above and below by relatively weak forces. The remarkable thing about graphene is that its crystalline structure is two-dimensional. In other words, the atoms in graphene are laid out flat, like billiard balls on a table. Each layer is made of hexagonal "rings" of carbon (like lots of benzene rings connected together, only with more carbon atoms replacing the hydrogen atoms around the edge), giving a honeycomb-like appearance. Since the layers themselves are just one atom high, you'd need a stack of about three million of these layers to make graphene 1mm thick!
Graphene or graphenes?
People talk about "graphene" the way they talk about "plastic," but it's important to remember that scientists are working on many different kinds of graphene-based materials (just like there are many different kinds of plastics), all of which are a little bit different and designed to do different things. In this article, I've followed the convention of calling the material "graphene," but it's as well to remember that this very new, fast-evolving substance has many different angles and aspects—and the word graphene will ultimately come to refer to a very wide range of different materials. One day, it may be common to talk about "graphenes" the way we now speak of "plastics."

Artwork: Graphene has a flat crystal lattice made from interlinked hexagons of carbon atoms (red blobs) tightly bonded together (black lines).
What is graphene like?
People are discovering and inventing new materials all the time, but we seldom hear about them because they're often not that interesting. Graphene was first discovered in 2004, but what's caused such excitement is that its properties (the way it behaves as a material) are remarkable and exciting. Briefly, it's super-strong and stiff, amazingly thin, almost completely transparent, extremely light, and an amazing conductor of electricity and heat. It also has some extremely unusual electronic properties.
General properties
Graphene is an amazingly pure substance, thanks largely to its simple, orderly structure based on tight, regular, atomic bonding, Carbon is a nonmetal, so you might expect graphene to be one too. In fact, it behaves much more like a metal (though the way it conducts electricity is very different), and that's led some scientists to describe it as a semimetal or a semiconductor (a material mid-way between a conductor and an insulator, such as silicon and germanium). Even so, it's as well to remember that graphene is extraordinary—and quite possibly unique.
Strength and stiffness
If you've ever scribbled with a soft pencil (something like a 4B), you'll know that graphite is horribly soft. That's because the carbon layers inside a stick of graphite shave off very easily. But the atoms within those layers are very tightly bonded so, like carbon nanotubes (and unlike graphite), graphene is super-strong—even stronger than diamond! Graphene is believed to be the strongest material yet discovered, some 200 times stronger than steel. Remarkably, it's both stiff and elastic (like rubber), so you can stretch it by an amazing amount (20-25 percent of its original length) without it breaking. That's because the flat planes of carbon atoms in graphene can flex relatively easily without the atoms breaking apart.
No-one knows quite what to do with graphene's super-strong properties, but one likely possibility is mixing it with other materials (such as plastics) to make compositesthat are stronger and tougher, but also thinner and lighter, than any materials we have now. Imagine an energy-saving car with super-strong, super-thin, super-light plastic body panels reinforced with graphene; that's the kind of object we might envisage appearing in a future turned upside down by this amazing material!
Thinness and lightness
Something that's only one atom thick is bound to be pretty light. Apparently, you could cover a football field with a sheet of graphene weighing less than a gram—although it's pretty unlikely anyone has actually tried! According to my quick calculations, that means if you could cover the entire United States with graphene, you'd only need a mass of around 1500–2000 tons. That might sound a lot, but it's only about as much as about 1500 cars—and it's completely covering one of the world's biggest countries!
Heat conductivity
As if super strength and featherweight lightness aren't enough, graphene is better at carrying heat (it has very high thermal conductivity) than any other material—better by far than brilliant heat conductors such as silver and copper, and much better than either graphite or diamond. Again, we're most likely to discover the benefit of that by using graphenes in composite materials, where we could use them to add extra heat-resistance or conductivity to plastics or other materials.
Electrical conductivity
This is where graphene starts to get really interesting! Materials that conduct heat very well also conduct electricity well, because both processes transport energy using electrons. The flat, hexagonal lattice of graphene offers relatively little resistance to electrons, which zip through it quickly and easily, carrying electricity better than even superb conductors such as copper and almost as well as superconductors (unlike superconductors, which need to be cooled to low temperatures, graphene's remarkable conductivity works even at room temperature). Scientifically speaking, we could say that the electrons in graphene have a longer mean free path than they have in any other material (in other words, they can go further without crashing into things or otherwise being interrupted, which is what causes electrical resistance). What use is this? Imagine a strong, light, relatively inexpensive material that can conduct electricity with greatly reduced energy losses: on a large scale, it could revolutionize electricity production and distribution from power plants; on a much smaller scale, it might spawn portable gadgets (such as cellphones) with much longer battery life.
Electronic properties

Photos: Advances in nanotechnology, including the development of graphene, will drive faster, smaller, cheaper computers. Picture by courtesy of Argonne National Laboratory published on Flickr under a Creative Commons Licence.
Electrical conductivity is just about "ferrying" electricity from one place to another in a relatively crude fashion; much more interesting is manipulating the flow of electrons that carry electricity, which is what electronics is all about. As you might expect from its other amazing abilities, the electronic properties of graphene are also highly unusual. First off, the electrons are faster and much more mobile, which opens up the possibility of computer chips that work more quickly (and with less power) than the ones we use today. Second, the electrons move through graphene a bit like photons (wave-like particles of light), at speeds close enough to the speed of light (about 1 million meters per second, in fact) that they behave according to both the theories of relativity and quantum mechanics, where simple certainties are replaced by puzzling probabilities. That means simple bits of carbon (graphene, in other words) can be used to test aspects of those theories on the table top, instead of by using blisteringly expensive particle accelerators or vast, powerful space telescopes.
Optical properties
As a general rule, the thinner something is, the more likely it is to be transparent (or translucent), and it's easy to see why: with fewer atoms to battle, photons are more likely to penetrate through thin objects than thick ones. As you might expect, super-thin graphene, being only one atom thick, is almost completely transparent; in fact, graphene transmits about 97–98 percent of light (compared to about 80–90 percent for a basic, single pane of window glass). Bearing in mind that graphene is also an amazing conductor of electricity, you can start to understand why people who make solar panels, LCDs, and touchscreens are getting very excited: a material than combines amazing transparency, superb electrical conductivity, and high strength is a perfect starting point for applications like these.
Impermeability
Sheets of graphene have such closely knit carbon atoms that they can work like super-fine atomic nets, stopping other materials from getting through. That means graphene is useful for trapping and detecting gases—but it might also have promising applications holding gases (such as hydrogen) that leak relatively easily from conventional containers. One of the drawbacks of using hydrogen as a fuel (in electric cars) is the difficulty of storing it safely. Graphenes, potentially, could help to make fuel-cell cars running on hydrogen a more viable prospect.
How do we make graphene?

Photo: Vapor deposition is used to create a layer of graphene on another surface (known as a substrate). Picture by Warren Gretz courtesy of US Department of Energy/National Renewable Energy Laboratory (DOE/NREL).
Take a pencil and some sticky tape. Stick the tape to the graphite, peel it away, and you'll get a layer of graphite made up of multiple layers of carbon atoms. Repeat the process very carefully, over and over again, and you'll (hopefully) end up with carbon so thin that it'll contain just one layer of atoms. That's your graphene! This rather crude method goes by the technical name of mechanical exfoliation. An alternative method involves loading up a super-precise atomic force microscope with a piece of graphite and then rubbing it very precisely on something so that single layers of graphene flake off, a bit like graphite from a pencil lead only one layer at a time. Techniques like this are fiddly and intricate and explain why graphene is currently the most expensive material on the planet!
These methods are fine for making tiny test samples of graphene in a laboratory, but there's no way we could make graphene like this on the kind of industrial scale on which it's likely to be required. So how do you make lots of graphene? One approach is to put an organic (carbon-based) gas such as methane into a closed container with something like a piece of copper in the bottom, then monkey with the temperature and pressure until a layer of graphene is formed on it. Because the graphene is formed by depositing layers of a chemical from a gas (vapor), this method is called chemical vapor deposition (CVD). Another approach involves growing crystals of graphene starting from a carbon-rich solid, such as sugar.
How can we use graphene?
We can answer that question in at least three different ways. First, because graphene has so many excellent properties, and because all those properties probably aren't needed in the same material (for the same applications), it makes sense to start talking about different types of graphene (or even different graphenes) that are being used in different ways or being optimized for particular purposes. So we're likely to see some graphenes being developed for structural uses (in composites materials), some being optimized to make the most of their extraordinary electron-carrying properties (for use in electronic components), others where we make the most of low-resistivity (in energy-saving power systems), and still others where excellent transparency and electrical conductivity are the important things (in solar cells and computer displays).

Photo: Computer memory chips like this might become smaller and faster if graphene replaces the silicon we currently use.
Second, we can see graphene as an exciting replacement for existing materials that have been pushed to their physical limits. Silicon transistors (the switching devices used as memories and "decision-making" logic gates in computers), for example, have consistently become smaller and more powerful over the last few decades, following a trend known as Moore's law, but computer scientists have long expressed concerns that the same rate of progress can't continue as we approach basic limitations imposed by the laws of physics. Some scientists are already imagining smaller and faster transistors in which silicon is replaced by graphene, taking computer devices even closer to the absolute limits of physics. In theory, we could use graphene to make ballistic transistors that store information or switch on and off at super-high speeds by manipulating single electrons. In much the same way, graphene could revolutionize other areas of technology constrained by conventional materials. For example, it could spawn lighter and stronger airplanes (by replacing composite materials or metal alloys), cost-competitive and more efficient solar panels (replacing silicon again), more energy-efficient power transmission equipment (in place of superconductors), and supercapacitors with thinner plates that can be charged in seconds and store more energy in a smaller space than has ever previously been possible (replacing ordinary, chemical batteries entirely). Companies such as Samsung, Nokia, and IBM are already developing graphene-based replacements for such things as touchscreens, transistors, and flash memories, though the work is at a very early stage.
Third, and most exciting of all, is the likelihood that we'll develop all kinds of brand-new, currently unimaginable technologies that take advantage of graphene's amazing properties. In the 20th century, plastics didn't simply replace older materials such as metal and wood: for better or worse, they completely changed our culture into one where disposability and convenience overtook durability. If graphenes lead us to ultra-light, ultra-thin, strong, transparent, optically and electrically conducting materials, who knows what possibilities might lie ahead. How about super-lightweight clothes made of graphenes, wired to batteries, that change color at the flick of a switch? Or an emergency house built for disaster areas, with graphene walls so strong and light that you can fold it up and carry it in a backpack?
Our graphene future?
Is it full-steam ahead to a future where graphene rules the world? Maybe—or maybe not. It's important not to get carried away with the hype: most of the exciting work on graphene has so far been done on a very small scale in chemical and physics laboratories. Most of the research is still what we'd describe as "blue sky": it could be many years or even decades before it can be developed practically, let along cost-effectively. By the same token, it's still very early days for basic scientific research into graphene. Forgetting all the amazing applications for a moment, there's doubtless much more exciting science to emerge. For example, we don't yet know if graphene is the only material with a two-dimensional crystal lattice—or if similar but even more extraordinary materials are just waiting to be discovered. One thing we do know is that this is a very exciting time for materials science!
Who discovered graphene?

Photos: The discovery of carbon nanotubes in 1991 helped spur researchers on to produce the first sample of graphene in 2004. Picture of aligned carbon nanotubes by Junbing Yang courtesy of Argonne National Laboratorypublished on Flickr under a Creative Commons Licence.
Scientists have been puzzling over graphene for decades. Back in 1947, Canadian physicist Philip Wallace wrote a pioneering paper about the electronic behaviour of graphite that sparked considerable interest in the field. Nobel-Prize winning chemist Linus Pauling was speculating about how flat, single layers of carbon atoms would behave as long ago as 1960. In 1962, such materials were named "graphene" by German chemist Hanns-Peter Boehm, who had spotted them under his electron microscope the year before.
Theoretical research into graphene continued for the next four decades, boosted in the 1980s and 1990s by the discoveries of fullerenes (effectively, graphene curled up into balls) and carbon nanotubes (graphene folded into a pipe). Even so, no-one could ever actually make the stuff in practice; graphene was only produced in a laboratory in 2004, by Russian-born scientists Andre Geim and Konstantin Novoselov working at the UK's University of Manchester. They made graphene by using pieces of sticky tape to pull off flakes of graphite, then folding the tape and pulling it apart to cleave the graphite into even smaller layers. Eventually, after a great deal of work, they were amazed to find they had some bits of graphite only one atom thick—graphene, in other words.
Four years later, the Manchester team managed to create a graphene transistor just one atom thick and ten atoms wide. The same year, workers at Rice University in the United States built the first graphene-based flash memory. In recognition of the huge importance of their work, Geim and Novoselov were awarded the 2010 Nobel Prize in Physics.

